Introduction
The Market for GMP Testing Services is gaining considerable momentum with growing regulatory pressure, rapid development of pharmaceutical and biopharmaceuticals, and a heightened focus on product quality and patient safety. GMP testing services play a pivotal role in ensuring that products are consistently manufactured and controlled according to quality criteria. As drug development pipelines expand and shrink and contract manufacturing companies (CMOs) gain significance, third-party GMP testing services are picking up pace globally.
GMP Testing Service Market Dynamics
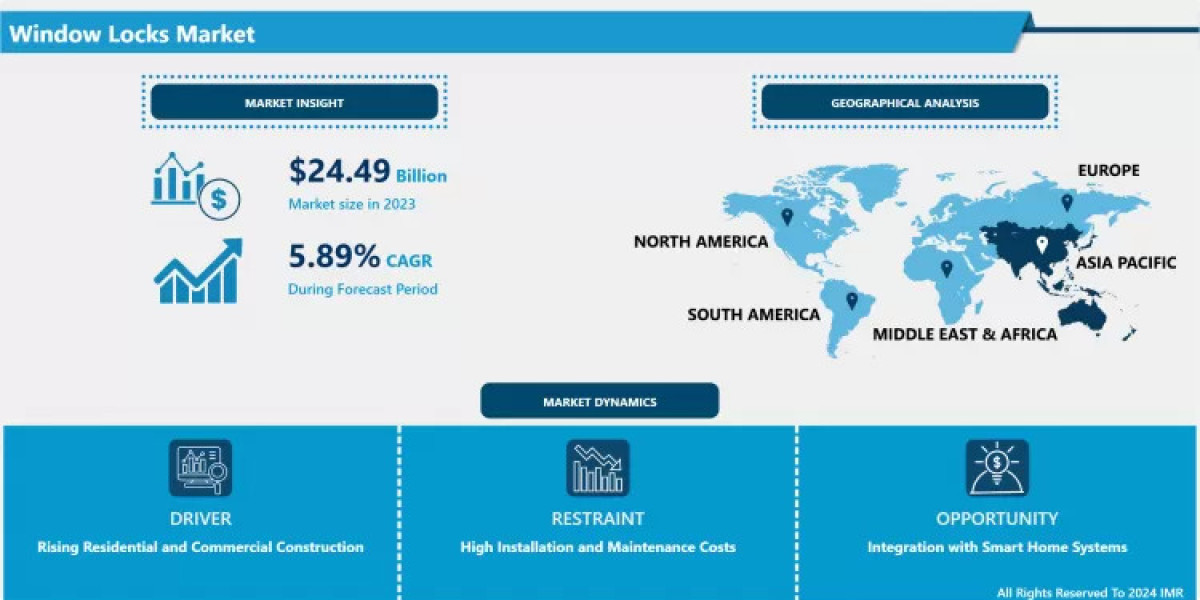
The market size of the GMP testing service is expected to grow to US$ 2.64 billion by 2031 from US$ 1.57 billion in 2023. The market is expected to grow at a CAGR of 6.7% during 2023–2031. Technical innovations in analysis techniques and hardware will continue to be significant market trends. Growth is driven by growing demand for outsourced quality control, expanding pharmaceutical R&D, and globalized supply chains for drugs. Regulatory agencies such as the FDA, EMA, and WHO are increasing frequency of inspections and raising GMP standards, compelling the manufacturers to go out and look for good partners to test. However, divergence among global regulatory systems and a lack of properly qualified technical professionals create hurdles for the market.
GMP Testing Service Market Growth Drivers
1. Stringent Regulatory Compliance Requirements
Global regulatory bodies are adopting more stringent GMP guidelines, which are forcing manufacturers to hire certified third-party testing laboratories to remain compliant and avoid product recalls.
2. Growth in Pharmaceutical and Biologics Development
With the growing number of small molecule drugs, biologics, and biosimilars, there is also growing demand for comprehensive GMP-conformant testing across the entire product life cycle.
3.Outsourcing and Contract Manufacturing Trends
As a way of simplifying operations and reducing in-house expenses, pharmaceutical firms more and more outsource quality control testing to third-party GMP-accredited support organizations.
4.Rise of Personalized Medicine and Advanced Therapies
Cell and gene therapies, requiring highly controlled and validated processes, are contributing substantially to the need for tailored GMP testing solutions.
5. Globalization of Drug Supply Chains
As drug companies have operations across multiple geographies, GMP testing services are essential to ensure consistency and adherence to regional compliance requirements.
Get Sample PDF Guide :- https://www.theinsightpartners.com/sample/TIPRE00029684
Innovations That Are Transforming the GMP Testing Service Market
• Automated Analytical Platforms
Artificial intelligence and automation are augmenting the precision and velocity of microbiological and analytical testing, lowering turnaround time and reducing the possibility of human error.
•Data Integrity and Digital Compliance Tools
The adoption of electronic lab notebooks (ELNs), Laboratory Information Management Systems (LIMS), and blockchain-based traceability tools improves transparency and audit-readiness.
•Sophisticated Microbial Testing Techniques
Next-generation sequencing (NGS) and rapid microbial detection methods are gaining popularity to provide faster and more precise sterility assurance.
•Integrated Quality Management Systems (QMS)
Service providers are investing in end-to-end quality systems that embrace risk assessment, validation, and document control within an integrated workflow.
•Tailored and Scalable Testing Solutions
GMP testing services specialized to nascent biotechs, startups, and complex modalities like mRNA treatments and nano-formulations are broadening the customer base.
GMP Testing Service Market Key Players and Recent Developments
1.Eurofins Scientific
Innovation: Advanced environmental monitoring via AI-driven platforms and blockchain to ensure data integrity in food safety testing.
2. North American Sciences Associates LLC
Innovation: Regulated compliance solutions developed by artificial intelligence that allow automated filings with numerous global jurisdictions.
3. Almac Group
Innovation: Virtual clinical trial platforms based on digital twins for efficient patient recruitment and data collection optimization.
4. PPD Inc
Innovation: IoT sensor-enabled smart, 3D-printed clinical trial kits offering real-time monitoring.
5. Intertek Group Plc
Innovation: Developing Green Chemistry Index™ to evaluate product manufacturing process sustainability metrics.
6. Nelson Laboratories
Innovation: Next-generation sequencing technology-based quick microbial identification systems for improved turnaround times.
GMP Testing Service Market Growth Opportunities
•Rising Demand from New Biopharma Participants
Start-up and mid-sized biopharma firms without in-house GMP facilities are seeking service providers for end-to-end quality testing services.
•Asia-Pacific and Latin America Expansion
Pharmaceutical manufacturing is increasing within these regions and offering lucrative chances for GMP testing laboratories based locally.
•Cell and Gene Therapy Quality Testing
Demand for highly specialist GMP test services is growing with the burgeoning pipeline of ATMPs (Advanced Therapy Medicinal Products).
•Stability and Shelf-Life Studies
As regulatory demands encircle longer-term stability information, service suppliers are offering enhanced real-time and accelerated stability tests.
•Digital Transformation and Smart Labs
Smart lab technology investments, automation, and cloud-based quality systems are gearing up providers for long-term operating efficiency and compliance.
Conclusion
The GMP Testing Service Market is poised for long-term expansion as pharmaceutical and biotechnology industries embrace outsourcing, precision medicine, and globalized operations. Exemplary GMP testing not only confirms regulatory adherence but also instills trust in drug safety and efficacy. Technology innovators, venturing into expanding markets, and tailored services for niche therapeutic classes will continue to dominate this evolving environment.